Structural biology of natural killer cell receptors and their interactions with ligands in health and disease
Contact person and project supervisor: RNDr. Ondřej Vaněk, PhD., ondrej.vanek@natur.cuni.cz
https://www.natur.cuni.cz/chemistry/biochem/research-1
Project summary:
Structural description of protein-protein interactions is the cornerstone of knowledge enabling detailed molecular understanding of processes such as receptor-ligand recognition within immune system, potentially leading to a design of targeted immunotherapeutics or drugs in general. Precise analysis of such interacting systems usually relies on X-ray structural analysis which is, however, dependent on successful preparation of diffracting crystals of the given protein complex – a task significantly more difficult for low-affinity or membrane protein complexes. On contrary, biophysical methods such as AUC, ITC, SPR, MST or chemical cross-linking and hydrogen/deuterium exchange mass spectrometry or small-angle X-ray scattering enable to study protein interactions directly in solution.
Combination of these techniques will be used for detailed study and molecular description of interactions of selected human and rodent natural killer cell receptors with their newly described protein ligands. The affinity of some of these protein complexes is rather low, as is often the case in immune recognition, thus making them very challenging for structural studies. At the same time, we are interested in understanding of how these interaction take place on the cell surface and trigger natural killer cell activation, or how these receptor:ligand complexes assemble within the immune synapse on the membrane of a living cell which brings to super-resolution microscopy techniques and single molecule localization microscopy using supported lipid bilayers reconstituted systems.
This research project is currently supported by Czech Science Foundation grants 18-10687S and 20-13029S.
Profile of an ideal candidate:
MSc. or equivalent in Biochemistry, Molecular Biology or a related field (required), good knowledge of English. Experience with DNA cloning and recombinant protein expression and purification, or with protein-related biophysical and structural biology methods is an advantage.
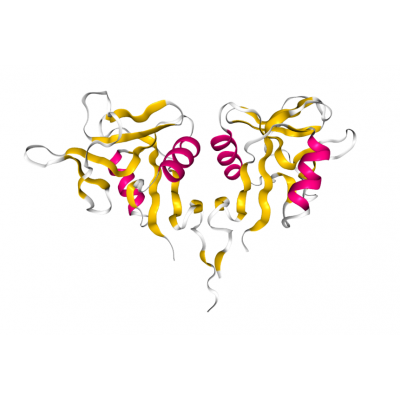
Crystal structure of the extracellular domain of human CD69 receptor solved at 1.37 Å resolution (PDB 3HUP).
Deadline is closed